
Saving Lungs Saving Lives
Cutting edge respiratory drug delivery
Fighting Cancer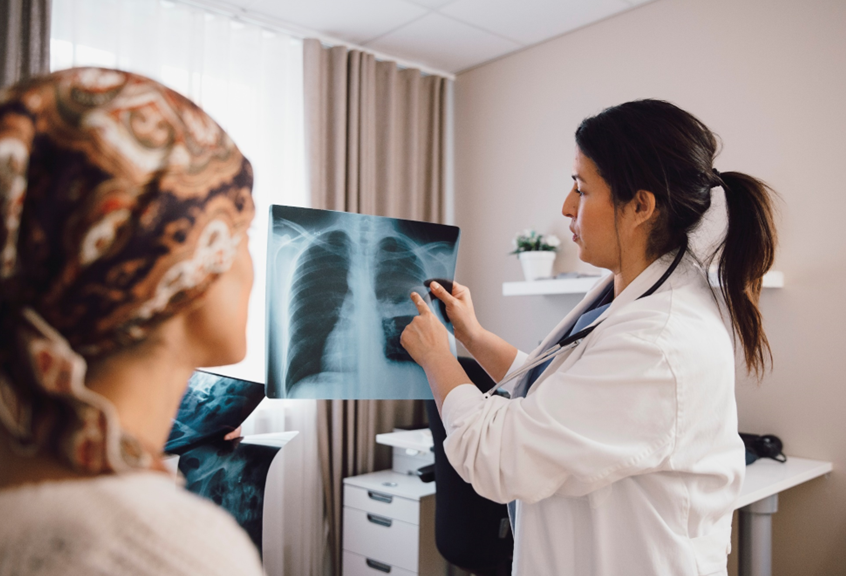
The pharmaceutical standard of care for lung cancer is primarily intravenous chemotherapy drugs, which have limited efficacy because of low drug concentration delivered to the lungs due to blood volume dilution.
Quench Medical’s inhalation technology delivers the drug directly to tumor tissues in the lung thereby enhancing its efficacy due to increased local drug concentration in the lung, and decreased systemic toxicity due to decreased systemic drug levels in the circulation.
Our new method delivers the chemotherapeutic drug via dry powder inhalation to reach lung tumors directly, in lower doses, in order to maximize the effectiveness and safety of the cancer treatment.

Founder & CEO

CFO

Director

Director
Directly targeting lung tumors with inhaled therapeutic to significantly increase survival while minimizing systemic toxicity
Unprecedented efficient delivery of therapeutic to the deep undertreated small airways to reduce inflammation and costly exacerbations
Deep penetration of therapeutic to treat infection and inflammation with high efficiency to increase healthspan